STRUCTURAL INVESTIGATIONS AND STRUCTURE-ACTIVITY RELATIONSHIPS IN A SERIE OF NEW ANTIBIOTIC 16- MEMBERED MACROLIDES
DOI:
https://doi.org/10.4314/jfas.1355Keywords:
16-membered macrolide, SAR, molecular mechanics, molecular dynamics, Boltzmann distributionAbstract
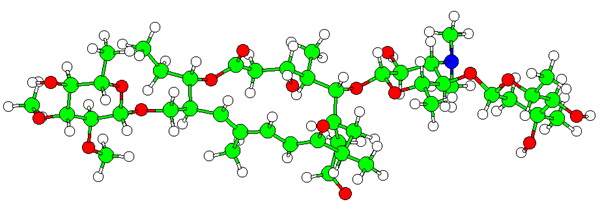
This study is a fundamental research on the structure- activity relationships in 16-membred macrolides. It is based on the molecular modelling (molecular mechanics, molecular dynamics, distribution de Boltzmann, PM3, SAR,). We defined the structural motives intervening in antibiotic macrolide properties. The compounds substituted in positions (C2, C4, C8, and C15) are the most stables and the less stable is substituted in C12. The rokitamycine which has the most elevated value of partition coefficient: 3.06. This antibiotic is lipophilic, so it has good permeability across the biological membrane, better fixation on plasma proteins and elimination by metabolic route.
Downloads
References
Brandena K. V., and Verbovenb S., Comput. Biol. Chem, 2009, 33.
Grant G., Richards W., ‘’Computational Chemistry’’, Oxford Chemistry Primers, Oxford, 1995.
Bakir M., and Gyles C., J. Mol. Structr., 2009, 918,138.
Pajak J., Maes G., Borggraeve W. M., Boens N., and Filarowski A., J. Mol. Structr. 2008, 880, 86.
Leeuw N. H., Mkhonto D., and Catlow C. R. A., J. Phys. Chem. B, 2003, 107, 1.
Mosier P. D., and Jurs P. C., J. Chem. Inf. Comput. Sci., 2002, 42, 1460.
Sánchez D. S., Muner D. S., Porcar N. S., Archivos de Bronconeumología (English Edition), 2010,46, 244-254.
Hlavica P., Biotechnology Advances, 2009, 27,103.
Belaidi S., Laabassi M., Grée R., and Botrel A., Rev. Roum. Chem. 2005, 50, 759.
Ouassaf M., Belaidi S., Lotfy K., Daoud I., Belaidi H., J. Bionanosci., 2018, 12, 26-36.
Dermeche K., Tchouar N., Belaidi S., Salah T., J. Bionanosci.2015, 9,395-400
Ouassaf M., Belaidi S., Khamouli S., Belaid H., Chtita S., Acta Chim. Slov.2021, 68, 289–303
VanBambeke F., Verhaegen J., Tyteca D., Auckenthaler R., and Tulkens P. M., Louv. Med., 2000, 119, 259.
Kaisalo L., thèse de doctorat, Université d’Helsinki, 2002, pp. 11.
Almi Z., Belaidi S., Segueni L., Rev. Theor. Sci. 2015, 3, 264-272
Kerassa A., Belaidi S., Harkati D., Lanez T., Prasad O., Sinha L., Rev. Theor. Sci., 2016, 4, 85-96
Deleu M., thèse de doctorat es sciences, FUSAGx, Belgique, 2000.
Statistical Physics. Course of Theoretical Physics. Vol. 5 (3 ed.). Oxford: Pergamon Press,1980
HyperChem (Molecular Modeling System) Hypercube, Inc., 1115 NW 4th Street, Gainesville, FL 32601; USA, 2009.
C.S.Chem 3D Ultra (Molecular modeling and Analysis), Cambridge Soft Corporation, 875, Massachusetts, Avenue Cambridge, Massachusetts, 02139 U.S.A, 2007.
Hocquet A., Act. Chim. 1997, 7, 24.
Allinger N. L., Zhou X., and Bergsma J., J. Mol. Structr. (Theochem), 1994, 312, 69.
Allinger N L., J. Am. Chem. Soc., 1977, 99, 8127.
Belaidi S, Omari M, Lanez T and Dibi A, J. Soc. Alger. Chim., 2004,14, 27-39
Van Bambeke F., Tyteca D., Ouadhriri Y., Tulkens P M., Louv. Med., 1999, 118, 43
Belaidi S., Dibi A., and Omari M., Turk J., Chem., 2002, 26, 491.
Belaidi S. Almi Z. , Bouzidi D., J. Comput. Theor. Nanosci. 2014, 11, 2481-2488
Belaidi S., Mazri R., Belaidi H., Lanez T., Bouzidi D., Asian J. Chem., 2013, 25, 9241-9245
. Van Bambeke F., Tulkens P M., Med. Mal. Infect., 2009 , 39, 483-92.
. Morimoto S., Nagate T., Sugita K., Ono T., Numata K., Miyachi Y., Omura S., J. Antibiot. Tokyo, 1990, 43, 295
. Miller K J., J. Am. Chem. Soc., 1990, 112, 8533.
. Yavorski B., and Detlaf A., ‘’Aide-mémoire de physique’’, p.376, Editions Mir, Moscou, 1980.
Khennoufa A, Bechki L, Lanez T, Lanez E, Zegheb N. Spectrophotometric, voltammetric and molecular docking studies of binding interaction of N-ferrocenylmethylnitroanilines with bovine serum albumin. Journal of Molecular Structure, 2021, 15, https://doi.org/10.1016/j.molstruc.2020.129052
Lanez T, Henni M. Antioxidant activity and superoxide anion radical interaction with 2-(ferrocenylmethylamino) benzonitrile and 3-(ferrocenylmethylamino) benzonitrile J. Iran. Chem. Soc., 2016, 13 (9), 1741-1748, https://doi.org/10.1007/s13738-016-0891-1
Lanez T, Benaicha H, Lanez E, Saidi M. Electrochemical, spectroscopic and molecular docking studies of 4-methyl-5-((phenylimino)methyl)-3H- and 5-(4-fluorophenyl)-3H-1,2-dithiole-3-thione interacting
with DNA. Journal of Sulfur Chemistry, 2018, 39(1), 76-88, https://doi.org/10.1080/17415993.2017.1391811
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Fundamental and Applied Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.


